Research
Multiple DNA repair pathways protects cells against the continuous threat of DNA damage, to prevent severe consequences such as cancer and aging. In our lab, we study the regulation and molecular mechanism of nucleotide excision repair (NER), transcription-coupled repair and interstrand crosslink repair, in relation to disease and cancer therapy.
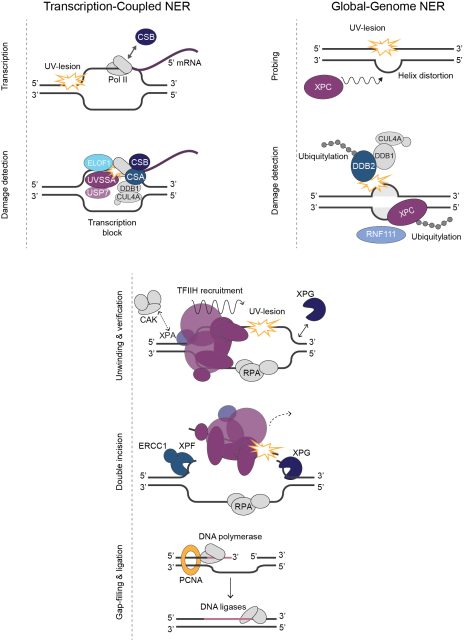
NER (see figure) is a versatile DNA repair pathway that recognizes lesions that distort the DNA helix. NER is initiated by damage detection throughout the genome, i.e. global genome NER, or by stalling of RNA polymerase II on a lesion, i.e. transcription-coupled NER. Damage detection leads to a sequential, multi-step mechanism that verifies the presence of damage, cuts out the damaged DNA strand and synthesizes a novel DNA strand. Detailed information on the mechanisms of GG-NER and TC-NER and the relation to cancer, aging and hereditary disease can be found in this and this elaborate review. We also published elaborative reviews describing the function and disease mechanisms of NER proteins TFIIH and XPG.
We study the molecular mechanism of DNA repair by means of live cell imaging, cell biology and molecular genetics techniques in combination with proteomics. For our studies, we make use of different model systems, including mammalian cells and organoids, and the model organism C. elegans. We investigate the molecular mechanism of NER, transcription-coupled DNA repair and interstrand crosslink repair, by studying the activity and regulation of individual proteins, and by studying how their deficiency leads to disease. Furthermore, we study DNA repair organization in vivo, in different cell types of C. elegans, and in the context of chromatin, i.e. by studying how chromatin remodeling factors are involved in DNA repair. Also, we study how NER and other DNA repair mechanisms protect cancer cells against chemotherapeutic drugs, with the ultimate goal of being able to improve the efficiency of chemotherapeutics drugs.